Applying Density Functional Theory to Common Organic Mechanisms: A Computational Exercise
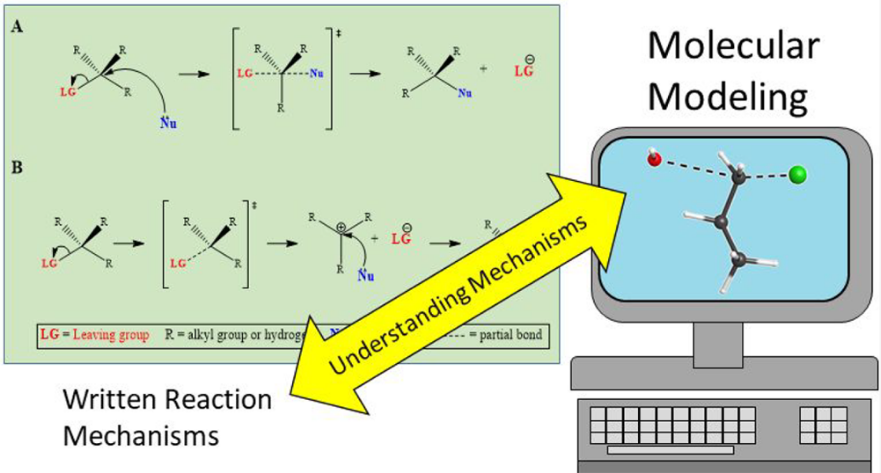 A computational experiment investigating common organic chemistry mechanisms has been developed and implemented in a junior/senior-level physical chemistry laboratory course at two institutions. Students investigated various reactions that proceed via SN1, SN2, E1, and E2 mechanisms using hybrid Density Functional Theory (DFT). Our pre/post-assessments indicate that students at both institutions were able to better visualize and interpret the 3D representation of transition states, stepwise reaction mechanisms, and reaction coordinate diagrams of the aforementioned reactions.
A computational experiment investigating common organic chemistry mechanisms has been developed and implemented in a junior/senior-level physical chemistry laboratory course at two institutions. Students investigated various reactions that proceed via SN1, SN2, E1, and E2 mechanisms using hybrid Density Functional Theory (DFT). Our pre/post-assessments indicate that students at both institutions were able to better visualize and interpret the 3D representation of transition states, stepwise reaction mechanisms, and reaction coordinate diagrams of the aforementioned reactions.
Learning Objectives
The learning objectives of the experiment are as follows:
- Better comprehension of mechanisms common in organic chemistry using a visual graphic.
- Understand and prepare reaction coordinate diagrams based on quantum chemical calculations.
- Tabulate and interpret the energetics of a chemical reaction via quantum chemical calculations.
- Application of quantum chemical models to chemical reactions using guided assistance.
- Learn how to identify transition states computationally using vibrational frequency calculations.
- Explain how thermochemical data was obtained using first-principles molecular software.
Reference
Jonathan P. Antle, Masashi W. Kimura, Stefano Racioppi, Corey Damon, Meredith Lang, Caitlyn Gatley-Montross, Laura S. Sánchez B., Daniel P. Miller, Eva Zurek, Adam M. Brown, Kellie Gast, and Scott M. Simpson, Journal of Chemical Education, 2023, 100 (1), 355-360, https://doi.org/10.1021/acs.jchemed.2c00935