What Does the Fluorescence Spectrum of an Excimer Tell You About Its Thermodynamics?
lab
thermo
spectroscopy
Timing: This experiment takes two or three lab periods, each at least three hours long.
Learning objectives
Content Objectives
Students will be able to:
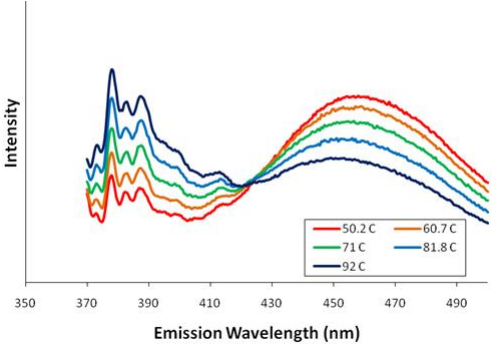
- identify the components of a monomer and excimer energy diagram; relate energy diagram lines, minima and spacing to experimental spectra and thermodynamic quantities
- use spectroscopic methods to quantitatively determine thermodynamic parameters and predict changes in spectra based on mathematical relationships
- become familiar with the operation of a fluorometer and temperature control apparatus.
Process Objectives
Students will be able to:
- explain the relationship between emission and excitation energies in fluorimetry.
- choose experimental parameters that allow determination of thermodynamic parameters from spectroscopic data.
- explain the relationship between spectral peaks, a pyrene monomer, and the formation of an excimer.
- apply mathematical models to data; use graphical methods to determine values for ΔS0 and ΔH0 by identifying the dependent and independent variables in a linear relation and relate these variables to measurable quantities in the lab.
- locating the desired properties from a linearized, graphed relationship using the slope and intercept.
Location
https://drive.google.com/file/d/1jKKGwUqmw2_DBXfNO2kVv-oZw_hIIfEe/view?usp=sharing
An instructor’s manual is available.
License
This work is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Status Reviewed
Reviewed