Encouraging Student Engagement by Using a POGIL Framework for a Gas-Phase IR Physical Chemistry Laboratory Experiment
What is this experiment about?
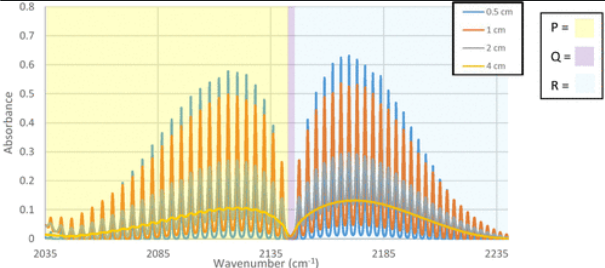
In this experiment, students collect and interpret IR spectra of a diatomic molecule (typically HCl or CO) with varying resolution. Students develop an energy level diagram and relate it to the rotational-vibrational spectra. They use spectral data to evaluate and refine quantum models, which are then used to extract molecular parameters including rotational constant and bond length. The lab presumes that the students are familiar with the fundamental ideas of spectroscopy (i.e. the energy of the radiation matches the energy change in the molecule being studied) and have had prior exposure to IR spectroscopy (likely in an organic chemistry course).
What do students do?
After collecting gas phase IR spectra of a small molecule at several different resolutions, students then try an initial mathematical model to fit the data and then work through the process of refining that model. Students consider the effect of resolution on the uncertainties of extracted parameters. Four additional optional cycles focus on isotopes, overtones, and statistical mechanics. Students can complete the non-optional portions of the experiment in two lab sessions. What equipment and supplies will you need? Data sets (HCl and CO) are available if an instructor wants to do this as a dry lab. For instructors collecting data, an IR instrument and a gas sample will be needed. Data analysis templates are available as an Excel spreadsheet and a Jupyter notebook.
What makes this experiment a physical chemistry experiment?
The experiment is often students’ first exposure to obtaining and analyzing spectra with sub-wavenumber resolution. Students use mathematical models to generate ro-vibrational energy diagrams and then use experimental data to update their diagrams and mathematical models. Students also acquire data at different spectral resolutions, enforcing the connection between resolution and the precision of the reported molecular constants. This experiment expands students’ understanding of how IR spectroscopy can be used beyond their previous classes where IR is used mainly for functional group identification.
And what makes it a POGIL-PCL experiment?
Students sketch a predicted IR spectrum and then go on to predict the effect of resolution on the spectrum. Students work in groups to collect spectra at different resolutions and then pool their data as a class. In groups and as a class, students are prompted to work through the process of refining model equations used to fit the spectra, using residuals to evaluate the fit. Students can then use the model to determine molecular parameters like the rotational constant and bond length, learning what information can be extracted from an IR spectrum.
Reference
Jordan P. Beck and Diane M. Miller, J. Chem. Educ., 2022, 99 (12), 4079–4084. https://doi.org/10.1021/acs.jchemed.2c00314
The Instructor’s Handbook with implementation details, sample data, and expected answers is available through the POGIL-PCL project.