What Are the Kinetic Parameters of a Heterogeneous Reaction?
What is this experiment about?
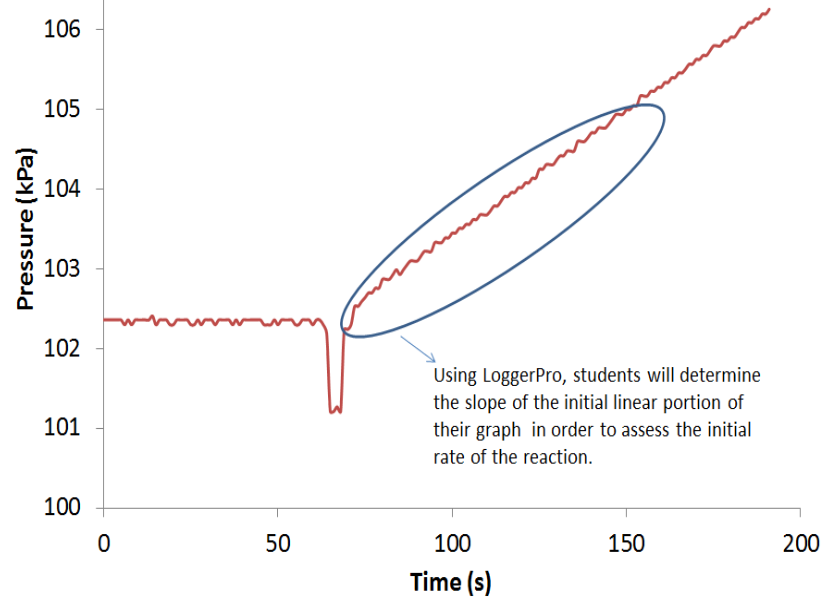
Students extend prior experience with chemical kinetics to the heterogeneous process of the oxidation of solid magnesium by dilute hydrochloric acid. Experimental design is a major component of the experiment. Students first do exploratory experiments to evaluate the measurable property and the form of solid magnesium they should use. Students then obtain rate data and calculate kinetics parameters. In the final cycle, students determine the activation energy.
What do students do?
In the first cycle, students react several forms of solid magnesium with dilute hydrochloric acid and measure the initial rate of hydrogen gas production. Data analysis helps identify the measurable property of magnesium that determines the reaction rate. They then choose the form of magnesium they should use for the remainder of the experiment. The second cycle introduces the rate law for the experiment. Students perform multiple kinetics runs varying the appropriate measurable properties of the solid magnesium and the dilute hydrochloric acid. Questions guide students in determining the kinetics parameters. A final cycle has students review the Arrhenius equation and design and perform experiments to evaluate the activation energy.
What equipment and supplies will you need?
- Solid magnesium (required: ribbon; at least one of: powder, turnings, rod)
- 1M HCl(aq) that students can dilute for use in their experiments
- Gas pressure sensor, such as from Vernier
- Constant temperature bath (room temperature to 45°C is sufficient)
- 125 mL Erlenmeyer flask as the reaction vessel, volumetric glassware, balances, thermometers
What makes this experiment a physical chemistry experiment?
The experiment illustrates how physical chemists make decisions about experiment parameters to obtain reliable results and build models for chemical behavior. Students first apply previous kinetics concepts to heterogeneous systems, making choices about forms of solid magnesium to use based on analysis of exploratory experiments. Once they have the rate law and kinetics parameters, they connect those to the Arrhenius equation that can describe the temperature dependence of the reaction rate.
And what makes it a POGIL-PCL experiment?
Students begin with experiment design, discussing the measurable properties that can affect reaction rate and making decisions about running experiments to explore those properties. They make predictions about how reactant properties or external conditions, such as temperature, affect reaction rates. In order to explore the possible parameters, including forms of solid magnesium, students share data and interact with their peers to obtain reliable results and conclusions. Students use graphical representations (initial rate determination) and mathematical models (the Arrhenius equation) in drawing conclusions.
Reference
Hunnicutt, S. S.; Grushow, A.; Whitnell, R. Guided-Inquiry Experiments for Physical Chemistry: The POGIL-PCL Model. J. Chem. Educ. 2015, 92 (2), 262–268. https://doi.org/10.1021/ed5003916.
The Instructor’s Handbook with implementation details, sample data, and expected answers is available through the POGIL-PCL project.
Highlight author: Rob Whitnell, Gilford College