Are the molecules that make a solution red big or small?
What is this experiment about?
 Students typically learn fundamental concepts of absorption spectroscopy, such as Beer’s Law, in their first chemistry courses. This experiment takes a classic experiment, the spectroscopy of cyanine dyes, reformulating it in guided inquiry and extending student work to develop a stronger understanding of the nature of molecular absorption of light. Students combine experimental measurement, quantum mechanical theory, and computer modeling through the use of PhET simulations to work through the complexity of what is often presented as a simple application of a particle-on-a-line model. Students should be familiar with Beer’s Law and the particle-on-a-line model.
Students typically learn fundamental concepts of absorption spectroscopy, such as Beer’s Law, in their first chemistry courses. This experiment takes a classic experiment, the spectroscopy of cyanine dyes, reformulating it in guided inquiry and extending student work to develop a stronger understanding of the nature of molecular absorption of light. Students combine experimental measurement, quantum mechanical theory, and computer modeling through the use of PhET simulations to work through the complexity of what is often presented as a simple application of a particle-on-a-line model. Students should be familiar with Beer’s Law and the particle-on-a-line model.
What do students do?
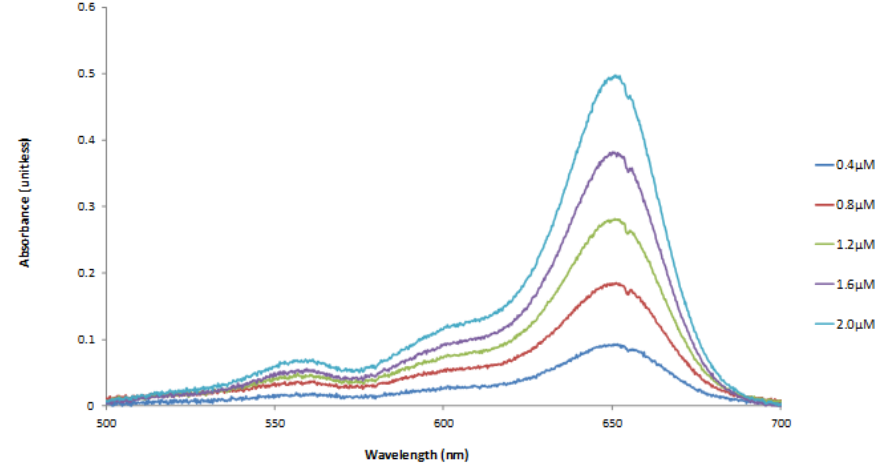
The full experiment consists of four experimental cycles. Students can first either use spectroscopy of cyanine dyes review or apply Beer’s Law in examining the relation between absorbance and concentration, based on instructor assessment of their experience. They predict the spectra of dye mixtures and perform corresponding experiments as they often do not grasp the specific additive nature of Beer’s Law. Students then apply the particle-on-a-line model to their experimental data, specifically focusing on refining the meaning of the line length. Finally, students use PhET simulations to examine the effects of using finite, instead of infinite, potential energy wells. Completing all four cycles typically requires two three-hour lab periods.
What equipment and supplies will you need?
Visible light absorption spectrophotometer that can identify λmax in the 400-700 nm region Series of carbocyanine or thiacyanine dyes in methanol solution as described in the instructor handbook. These may seem expensive, but they will provide for many years of experiments. What makes this experiment a physical chemistry experiment? This experiment illustrates several tenets of physical chemistry. Students test Beer’s Law, which is often first presented as phenomenological. They explore how the law applies to more complex systems such as a mixture of absorbing species, and they look at the interplay between theoretical models and experimental results. They see how the model parameters (length of a box) require exploring interpretations in terms of molecular properties. And they also observe how modifying the model can lead to better agreement with experiment and further insight into molecular behavior.
And what makes it a POGIL-PCL experiment?
Students make predictions about the behavior of systems of absorbing molecules, testing those predictions against experimental results they obtain. In doing so, students engage in experimental design and decision-making to determine dye concentrations to use, assign dyes to different teams to ensure reproducible results, and share data among the class to obtain a complete overview of the results. Students use graphical representations to explore the results and apply, modify, and extend mathematical models to obtain a more complete description of π-electron behavior in dyes.
Reference
Lead Author: Rob Whitnell, Guilford College
The Instructor’s Handbook with implementation details, sample data, and expected answers is available through the POGIL-PCL project.
Intellectual Property and Copyrights statement.