How Can We Make Stew With These Beans?
What is this experiment about?
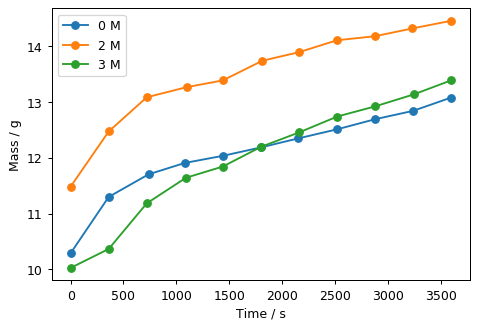
Experiments for physical chemistry lab courses often address concepts and systems that students can rely on applying what they’ve seen previously instead of learning how to address an unfamiliar system. In this experiment, students study the rate at which chickpeas absorb water, a system that is familiar to to any cook, but new from the perspective of how a physical chemist would study the process. Equipped with chickpeas, salt water, and a balance, students observe the regularity of the absorption rate and the effect of varying salt concentration. To model the data, students use non-linear fitting techniques that can be done in Jupyter notebooks or Excel. The experiment is an excellent and procedurally simple introduction to how physical chemists approach new phenomena.
What do students do?
The experiment consists of three cycles that can be completed in two three-hour lab periods. Students first use a basic experimental protocol and decide several aspects of the experimental design: data collection frequency, salt concentrations, reproducibility, reliable mass measurement. Through data plotting and initial rate calculation, students draw conclusions about time dependence and salt concentration effects. They then analyze the Peleg equation that describes a wide range of food absorption processes. Students revise their experiment design and perform longer runs, constructing qualitative and quantitative comparisons to the Peleg equation. In the third cycle, students connect their results to osmosis concepts, extending their understanding of the underlying models in the post-experiment questions.
What equipment and supplies will you need?
- Chickpeas and stock NaCl solution, typically 3M
- Balances–even inexpensive ±0.1g pocket balances are sufficient to get good data
- Glassware, funnels, and other basic equipment that students can use for their experiment design
What makes this experiment a physical chemistry experiment?
This experiment provides a simple system that illustrates how physical chemists approach new phenomena. They explore qualitatively what the system does before trying to obtain detailed data. When given an equation that is claimed to fit the data, they look at appropriate limits to address its validity before doing more complex fitting. The Peleg equation introduces the common approach of non-linear fitting, allowing students to develop a reasonable interpretation for the underlying molecular processes.
And what makes it a POGIL-PCL experiment?
Students develop hypotheses about the factors that affect water uptake by dry food. They make predictions about the effect of salt concentration, and they test those predictions both qualitatively and quantitatively. They engage in decision-making and experiment design, in running the experiment and data analysis. They share responsibility as a class for the experiment design and share data to provide a wide range of experimental conditions. Students use graphical representations of data to explore qualitative and quantitative aspects and non-linear fitting of a hypothesized mathematical model.
Reference
Lead author: Maria Pacheco, Buffalo State College
The Instructor’s Handbook with implementation details, sample data, and expected answers is available through the POGIL-PCL project.
Highlight author: Rob Whitnell, Guilford College