An Investigation of the Temperature Dependence of a Monomer−Dimer Equilibrium Using UV−Vis and 1H NMR Spectroscopies
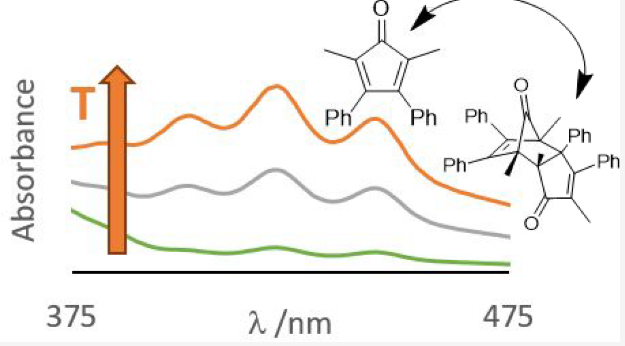
A laboratory experiment in which 1H NMR and UV−vis spectroscopies are applied to probe the temperature dependence of a monomer−dimer equilibrium involving a cyclopentadienone derivative is described and discussed. Details of the data analysis for extraction of equilibrium constants from the raw experimental data from both techniques are provided, leading to the determination of thermodynamic parameters (ΔrH° and ΔrS°) via van’t Hoff analysis. Fully analyzed datasets are provided in the Supporting Information to aid adaptation of the experiment or its use as a data analysis activity.
Learning Objectives
Skills
- Mass/moles/concentration calculations.
- Planning your work: time management and communication.
- Manipulation of data using a spreadsheet.
- Graphical analysis of data using a spreadsheet.
- Mathematical skills: manipulating and solving algebraic equations
- Locating and extracting information from research papers.
Objectives
- Obtain the UV-Vis spectra of solutions equilibrated at various temperatures
- Determine equilibrium constants for the Diels-Alder reaction at various temperatures from visible absorbance (A) data using the Beer-Lambert law.
- Determine thermodynamic parameters (ΔrH° and ΔrS°) for the Diels-Alder reaction using the van’t Hoff equation.
- Compare experimentally determined values of ΔrH° and ΔrS° with published values.
Citation
Laura M. Hancock, David J. McGarvey, and Daniela Plana, Journal of Chemical Education 2023 100 (3), 1283-1288, https://doi.org/10.1021/acs.jchemed.2c00917