Derivation of Morse Potential Function
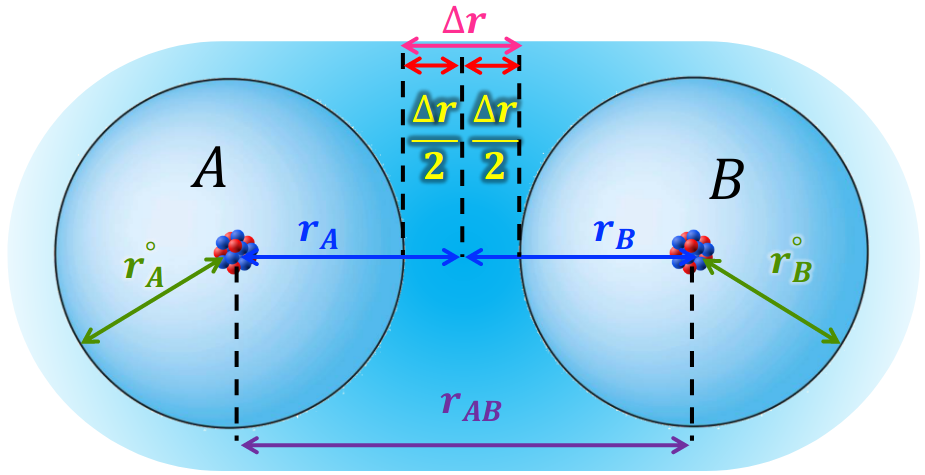
The Morse potential is widely used in chemistry to describe interatomic interactions. However, there is no explicit derivation for this empirical potential from physically meaningful atomic quantities. We show that the Morse potential can be derived from a simple atomic screened charge model, which accounts for the shielded nuclear charge by the electron density and exponentially de- cays with distance. The bond dissociation energy of a diatomic molecule is obtained by combining the quantum mechanical covalent and classical electrostatic interactions. The revealed connections between the parameters of the Morse potential, the Pauling bond order and electronegativity bridge the gap between the classical and quantum mechanical descriptions of chemical bonds. The proposed derivation and interpretation of the Morse potential in terms of atomic quantities such as electron-nuclear attraction energy and orbital exponents will be valuable in helping students to form a simple picture of chemical bond.
Reference
Mirzanejad A, Varganov S. Derivation of Morse Potential Function. ChemRxiv. Cambridge: Cambridge Open Engage; 2023; This content is a preprint and has not been peer-reviewed.
License
The content is available under CC BY NC ND 4.0 CreativeCommons.org